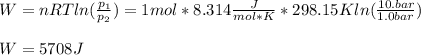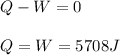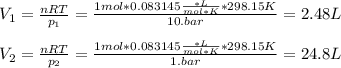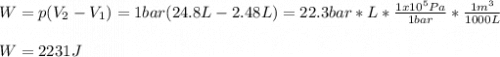Chemistry, 25.11.2020 02:30 twistedgamerhd12
One mole of an ideal gas expands reversibly and isothermally from 10. bar to 1.0 bar at 298.15K.
Required:
a. Calculate the values of w, q, âU and âH?
b. Calculate w if the gas were to have expanded to the same final state against a constant pressure of 1 bar.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:00
If a plot weight (in g) vs. volume (in ml) for a metal gave the equation y= 13.41x and r^2=0.9981 what is the density of the metal?
Answers: 2

Chemistry, 21.06.2019 23:00
At room temperature what happens to the average kinetic energy of the molecules of a solid, liquid, and a gas
Answers: 2

Chemistry, 22.06.2019 11:10
Which of the following shapes would represent a molecule with two bonded atoms and 3 lone pairs on only one of them , trigonal planar , bent , trigonal pyramidal , linear
Answers: 1

Chemistry, 22.06.2019 18:00
Which three statements represent the benefits of performing experiments using computer simulations?
Answers: 2
You know the right answer?
One mole of an ideal gas expands reversibly and isothermally from 10. bar to 1.0 bar at 298.15K....
Questions



Mathematics, 16.10.2019 07:30

Mathematics, 16.10.2019 07:30

Mathematics, 16.10.2019 07:30





Mathematics, 16.10.2019 07:30




Computers and Technology, 16.10.2019 07:30

Mathematics, 16.10.2019 07:30

Social Studies, 16.10.2019 07:30

Computers and Technology, 16.10.2019 07:30

Mathematics, 16.10.2019 07:30


Mathematics, 16.10.2019 07:30