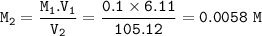Chemistry, 25.11.2020 05:30 rhettperkins
Calculate the Molarity when a 6.11 mL solution of 0.1 H2SO4 is diluted with 105.12 mL of water

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:40
Consider the nuclear equation below. 239/94 pu—-> x+ 4/2 he. what is x?
Answers: 2

Chemistry, 22.06.2019 11:30
Aperfume bottle is dropped in the corner of a room. the odor of the perfume can be detected on the other side of the room. which statement best describes this observation?
Answers: 2

Chemistry, 22.06.2019 14:30
The valence of aluminum is +3, and the valence of the chlorine is -1. the formula fir the aluminum chloride is correctly written as
Answers: 2

Chemistry, 22.06.2019 22:30
Amedication is given at a dosage of 3.000 mg of medication per kg of body weight. if 0.1500 g of medication is given, then what was the patient's weight in pounds (lbs)? there are 453.59g in 1 lb.
Answers: 2
You know the right answer?
Calculate the Molarity when a 6.11 mL solution of 0.1 H2SO4 is diluted with 105.12 mL of water...
Questions


Biology, 02.12.2020 01:00

Mathematics, 02.12.2020 01:00




Chemistry, 02.12.2020 01:00


History, 02.12.2020 01:00

Mathematics, 02.12.2020 01:00

Mathematics, 02.12.2020 01:00



Mathematics, 02.12.2020 01:00


Mathematics, 02.12.2020 01:00



Mathematics, 02.12.2020 01:00

Spanish, 02.12.2020 01:00