Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Drive down any three characteristic of modern periodic table
Answers: 1

Chemistry, 22.06.2019 14:00
Calculate the frequency of a wave in a spring toy. the wave has a speed of 1.1 meters per second and a wavelength of 0.1 meters. *
Answers: 2

Chemistry, 22.06.2019 15:30
The gulf stream is a warm water current that flows away from the equator to northern europe. witch of these does it cause. a. crashes of warm and cool water in the ocean b.colder climates near the equator c.large waves on the cost of europe d.warm climates in northern europe
Answers: 1

Chemistry, 22.06.2019 19:20
Consider hydrogen in an excited state n = 5n=5 that emits photons to reach the ground state. there are various possible transitions other than straight to the ground state that can occur; for example, it can drop to the n = 3n=3 state followed by the n = 3n=3 to the ground state transition. which of the possible transitions will result in the emission of a photon in the visible region?
Answers: 3
You know the right answer?
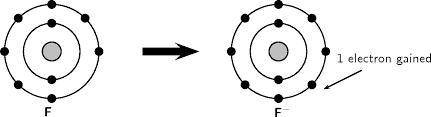
The fluorine ion, F, has one more electron than a neutral fluorine atom.
a) How many pea-halves do...
Questions


Advanced Placement (AP), 03.04.2020 03:43

English, 03.04.2020 03:43






Mathematics, 03.04.2020 03:43




Mathematics, 03.04.2020 03:43


Mathematics, 03.04.2020 03:44



Social Studies, 03.04.2020 03:44

Social Studies, 03.04.2020 03:44

Mathematics, 03.04.2020 03:44