Chemistry, 25.11.2020 14:20 cecilysimpson7521
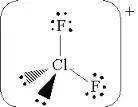
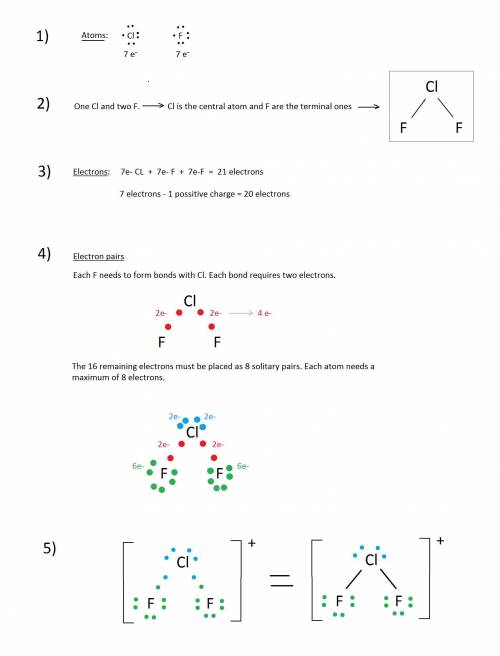
Draw the Lewis structure with the lowest formal charges for ClF2+ . Include nonzero formal charges and lone pair electrons in the structure.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 14:00
Anthracite is so hard and pure it is also referred to as a renewable resource metamorphic rock hot bituminous coal dirty fuel
Answers: 1

Chemistry, 22.06.2019 14:30
How does a noncompetitive inhibitor reduce an enzyme’s activity?
Answers: 1


Chemistry, 22.06.2019 20:00
Many free radicals combine to form molecules that do not contain any unpaired electrons. the driving force for the radical–radical combination reaction is the formation of a new electron‑pair bond. consider the chemical equation. n(g)+no(g)⟶nno(g) n(g)+no(g)⟶nno(g) write lewis formulas for the reactant and product species in the chemical equation. include nonbonding electrons. n(g)n(g) select draw rings more erase select draw rings more erase select draw rings more erase n no(g)
Answers: 1
You know the right answer?
Draw the Lewis structure with the lowest formal charges for ClF2+ . Include nonzero formal charges a...
Questions

English, 03.04.2020 04:12


Mathematics, 03.04.2020 04:12



Mathematics, 03.04.2020 04:13



Chemistry, 03.04.2020 04:13

Chemistry, 03.04.2020 04:13

History, 03.04.2020 04:13

History, 03.04.2020 04:13

Mathematics, 03.04.2020 04:13




English, 03.04.2020 04:13


English, 03.04.2020 04:13

Mathematics, 03.04.2020 04:13