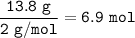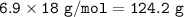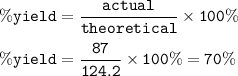Use the equation below to solve the problem that follows.
2H2 (g) + O2 (g) → 2H2O (g)
W...


Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:30
In 2002, the rare earth elements mine in mountain pass, california was closed because
Answers: 1

Chemistry, 22.06.2019 12:00
In a laboratory, 1.55mg of an organic compound containing carbon, hydrogen, and oxygen is burned for analysis. this combustion resulted in the formation of 1.45mg of carbon dioxide and .89 mg of water. what is the empirical formula for this compound?
Answers: 1

Chemistry, 22.06.2019 12:30
What metric units would you use to measure the thickness of a key
Answers: 3

You know the right answer?
Questions

Biology, 20.08.2019 07:30



Mathematics, 20.08.2019 07:30

Mathematics, 20.08.2019 07:30

English, 20.08.2019 07:30

Physics, 20.08.2019 07:30

Mathematics, 20.08.2019 07:30


Social Studies, 20.08.2019 07:30

Mathematics, 20.08.2019 07:30

Mathematics, 20.08.2019 07:30

Mathematics, 20.08.2019 07:30

Mathematics, 20.08.2019 07:30

Mathematics, 20.08.2019 07:30




Mathematics, 20.08.2019 07:30