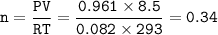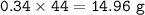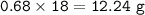Chemistry, 26.11.2020 03:00 kell22wolf
If 8.50 L of natural gas, which is essentially methane (CH4), undergoes complete combustion at 730 mm Hg and 20 degrees C, how many grams of each product are formed?
Grams of CO2=
Grams of H2O=

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:00
Astudent is asked to identify and element that is pale yellow brittle solid and does not conduct electricity. at which location in this periodic table would the element most likely be found?
Answers: 2

Chemistry, 22.06.2019 12:10
Achemistry student needs to standardize a fresh solution of sodium hydroxide. he carefully weighs out of oxalic acid , a diprotic acid that can be purchased inexpensively in high purity, and dissolves it in of distilled water. the student then titrates the oxalic acid solution with his sodium hydroxide solution. when the titration reaches the equivalence point, the student finds he has used of sodium hydroxide solution.calculate the molarity of the student's sodium hydroxide solution. be sure your answer has the correct number of significant digits.
Answers: 1


Chemistry, 22.06.2019 15:50
Elements in group 2 are all called alkaline earth metals. what is most similar about the alkaline earth metals?
Answers: 1
You know the right answer?
If 8.50 L of natural gas, which is essentially methane (CH4), undergoes complete combustion at 730 m...
Questions

Mathematics, 05.02.2021 21:40




English, 05.02.2021 21:40



English, 05.02.2021 21:40



Arts, 05.02.2021 21:40

Biology, 05.02.2021 21:40



Arts, 05.02.2021 21:40

Mathematics, 05.02.2021 21:40


Mathematics, 05.02.2021 21:40