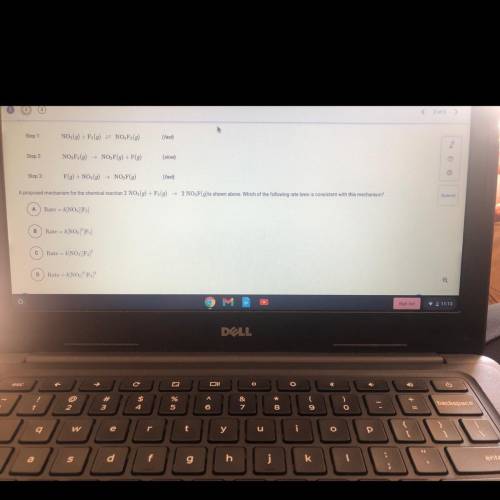
Step 1:
NO2(g) + F2(g) = NO, F2(g)
(fast)
Step 2
NO, F2(9)
NO, F(9) + F(g)<...

Chemistry, 26.11.2020 22:30 ineedhelp2285
Step 1:
NO2(g) + F2(g) = NO, F2(g)
(fast)
Step 2
NO, F2(9)
NO, F(9) + F(g)
(slow)
Step 3:
F(g) + NO (9) NO, F(g)
(fast)
A proposed mechanism for the chemical reaction 2 NO2(g) + F (9)
2 NO, F(g)is shown above. Which of the following rate laws is consistent with this mechanism?
A
Rate = k/NO2][F]
B
Rate = k[NO][F)
С
Rate -- k[NO] F2)
D
Rate
k[NO, F,

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 19:10
Imagine that you have produced several versions of lactase, each of which differs from normal lactase by a single amino acid. describe a test that could indirectly determine which of the versions significantly alters the three-dimensional shape of the lactase protein.
Answers: 2


Chemistry, 23.06.2019 00:00
What is the pressure of 0.500 moles of carbon dioxide gas in a 2.5 l tank and at a temperature of 301 k? (r=0.0821 l·atm/mol·k) 3.08 atm 1.2 atm 0.23 atm 4.01 atm 4.94 atm
Answers: 1

Chemistry, 23.06.2019 08:00
Which of the following notations would be the appropriate and final way to display the formula for magnesium chloride a. mgcl2 b. mg+2cl–1 c. mgcl2 d. mgcl
Answers: 2
You know the right answer?
Questions








History, 03.04.2020 19:13



Advanced Placement (AP), 03.04.2020 19:14

Mathematics, 03.04.2020 19:14



Mathematics, 03.04.2020 19:14







