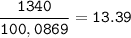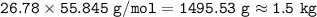Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:00
Suppose you have designed a new thermometer called the x thermometer. on the x scale the boiling point of water is 129 ? x and the freezing point of water is 13 ? x. part a at what temperature are the readings on the fahrenheit and x thermometers the same?
Answers: 1

Chemistry, 22.06.2019 15:20
Which description best characterizes the motion of particles in a solid?
Answers: 2

Chemistry, 22.06.2019 16:00
He table below gives the atomic mass and relative abundance values for the three isotopes of element m. relative abundance (%) atomic mass (amu) 78.99 23.9850 10.00 24.9858 11.01 25.9826 what is the average atomic mass (in amu) of element m? 2.86 5.36 24.30 24.98
Answers: 2

Chemistry, 22.06.2019 19:00
How many liters of ethylene glycol antifreeze (c2h6o2), with a density of 1.100 g/l, would you add to your car radiator containing 15.0 kg of water if you needed to protect your engine to - 21.5°c? for water, kf = 1.86°c m -1.
Answers: 1
You know the right answer?
In the metallurgic industry one of the processes to get pure iron takes tree steps.
a) CaCO3→CaO +...
Questions


Mathematics, 29.05.2021 01:50





Mathematics, 29.05.2021 01:50

Mathematics, 29.05.2021 01:50


Mathematics, 29.05.2021 01:50


History, 29.05.2021 01:50

Mathematics, 29.05.2021 01:50

Biology, 29.05.2021 01:50

Arts, 29.05.2021 01:50


History, 29.05.2021 02:00

Mathematics, 29.05.2021 02:00

Mathematics, 29.05.2021 02:00