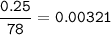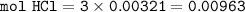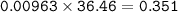Chemistry, 27.11.2020 02:00 carrieaj08
2. Al(OH),(s) + 3 HCl(aq) à 3 H2O(l) + AlCl3(aq). This reaction shows how aluminum hydroxide
in antacid tablets neutralizes hydrochloric acid in the stomach. A tablet containing 0.25 g of
aluminum hydroxide is ingested by a patient with 0.88 g of hydrochloric acid in their stomach. Is
this tablet sufficient to neutralize the acid in the patient's stomach? Explain using stoichiometric
calculations. [4 marks]
I

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Which of these properties, used alone, would be least useful in identifying most minerals? a. color b. luster c. streak d. density
Answers: 2


Chemistry, 22.06.2019 17:50
You exhale co2 which is produced during cellular respiration. co2 combines with the water in your blood's plasma to make up one half of the body's most important buffer pair, carbonic acid. the more physical activity you engage in, the more co2 your body is producing. you can see this by putting some of the cabbage indicator in a glass and then blowing bubbles into it through a straw. can you see a change in the color of the indicator?
Answers: 2

Chemistry, 23.06.2019 01:00
What is the chemical name of the compound ti2o3? use the list of polyatomic ions and the periodic table to you answer.
Answers: 1
You know the right answer?
2. Al(OH),(s) + 3 HCl(aq) à 3 H2O(l) + AlCl3(aq). This reaction shows how aluminum hydroxide
in ant...
Questions

Geography, 15.07.2021 05:40




Mathematics, 15.07.2021 05:40


History, 15.07.2021 05:40



English, 15.07.2021 05:40



Mathematics, 15.07.2021 05:40

Business, 15.07.2021 05:40

English, 15.07.2021 05:40

Spanish, 15.07.2021 05:40

English, 15.07.2021 05:40

Chemistry, 15.07.2021 05:40

Arts, 15.07.2021 05:40

Computers and Technology, 15.07.2021 05:40