
Chemistry, 27.11.2020 16:20 s0cial0bessi0n
A 6g sample of carbon allowed to burn in 20g of oxygen gas produce carbon dioxide. After the reaction, the mass of unreacted oxygen is 4 g. What mass of carbon dioxide was produced? show with steps

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:30
If 34.2 grams of lithium react with excess water, how many liters of hydrogen gas can be produced at 299 kelvin and 1.21 atmospheres? 2 li (s) + 2 h2o (l) yields 2 lioh (aq) + h2 (g)
Answers: 3

Chemistry, 22.06.2019 08:30
Which metal exist in liquid state and can be cut with knife ?
Answers: 2

Chemistry, 22.06.2019 13:00
Using the thermodynamic information in the aleks data tab, calculate the standard reaction free energy of the following chemical reaction: →+p4o10s6h2ol4h3po4s round your answer to zero decimal places.
Answers: 3

Chemistry, 22.06.2019 22:30
Molecular iodine, i2(g), dissociates into iodine atoms at 625 k with a first-order rate constant of 0.271 s−1. part a part complete what is the half-life for this reaction?
Answers: 3
You know the right answer?
A 6g sample of carbon allowed to burn in 20g of oxygen gas produce carbon dioxide. After the reactio...
Questions





History, 05.07.2019 16:30

History, 05.07.2019 16:30

Social Studies, 05.07.2019 16:30

Geography, 05.07.2019 16:30

Social Studies, 05.07.2019 16:30

Mathematics, 05.07.2019 16:30

English, 05.07.2019 16:30

Mathematics, 05.07.2019 16:30


Social Studies, 05.07.2019 16:30





Mathematics, 05.07.2019 16:30

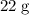 .
. :
: 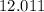 .
. :
: 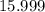 .
.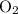 and
and 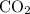 :
: .
.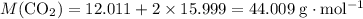 .
.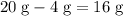 of
of 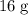 of
of 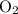 :
: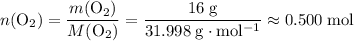 .
.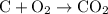 .
.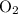 and
and 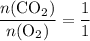 . For each mole of
. For each mole of 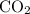 will be produced.
will be produced.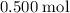 , of
, of 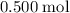 of
of 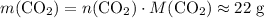 .
.

