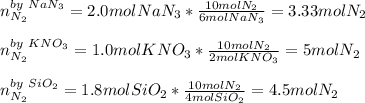Chemistry, 28.11.2020 03:20 isabellemaine
When an airbag expands in a vehicle, sodium azide reacts with potassium nitrate and silicon dioxide, releasing nitrogen gas and sodium potassium silicate (fine glass powder) 6 NaN3+ 2 KNO3 + 4 SiO210 N2+ 2 NaKSiO3+ 2 Na2SiO3 + 02 To conduct a similar reaction, 2.0 mol of NaN3, 1.0 mol of KNO3 and 1.8 mol of SiO2 are added. Which one is the limiting reactant? a) N2 b) O2 c) KNO3 d) SiO2

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:30
You are to give ampicillin with a recommended dose of 25mg/kg to a child with a mass of 29kg. if stock on hand is 250mg/capsule how many capsules should be given?
Answers: 1

Chemistry, 22.06.2019 04:50
Acompound contains c, h, and o atoms. when 1.130 g of the compound is burned in oxygen, 1.064 g co2 and 0.3631 g h2o are produced. what is the empirical formula of this compound?
Answers: 1

Chemistry, 22.06.2019 12:30
The melting point of sulfur is 115 °c and its boiling point is 445 °c. what state would sulfur be in at 200 °c?
Answers: 1

You know the right answer?
When an airbag expands in a vehicle, sodium azide reacts with potassium nitrate and silicon dioxide,...
Questions


English, 01.02.2020 04:43




Mathematics, 01.02.2020 04:43





History, 01.02.2020 04:43


Mathematics, 01.02.2020 04:43

English, 01.02.2020 04:43


Mathematics, 01.02.2020 04:43

Mathematics, 01.02.2020 04:43

Mathematics, 01.02.2020 04:43