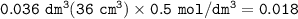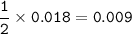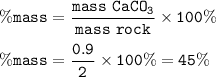Chemistry, 28.11.2020 22:30 nurmukhammada
A piece of rock has a mass of 2.00g. It contains calcium carbonate, but no other
substances. It neutralises exactly 36.0 cm of 0.500 moldmhydrochloric acid.
What is the percentage of calcium carbonate in the 2.00 g piece of rock?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:00
1) these are barrel shaped microtubules in most animal cells, that organize the spindles during cell division
Answers: 1

Chemistry, 22.06.2019 15:20
Water is initially present in a state where its molecules are far apart. during a change of state, its molecules slow down. which change of state has most likely taken place? from a gas to a liquid from a liquid to a gas from a solid to a liquid from a gas to a plasma
Answers: 1

Chemistry, 22.06.2019 20:00
There are two steps in the usual industrial preparation of acrylic acid, the immediate precursor of several useful plastics. in the first step, calcium carbide and water react to form acetylene and calcium hydroxide: cac2 (s) + 2h2o (g) → c2h2 (g) + caoh2 (s) =δh−414.kj in the second step, acetylene, carbon dioxide and water react to form acrylic acid: 6c2h2 (g) + 3co2 (g) + 4h2o (g) → 5ch2chco2h (g) =δh132.kj calculate the net change in enthalpy for the formation of one mole of acrylic acid from calcium carbide, water and carbon dioxide from these reactions. round your answer to the nearest kj .
Answers: 3

Chemistry, 22.06.2019 20:30
Which of the following is not true about the atomic model of substances?
Answers: 1
You know the right answer?
A piece of rock has a mass of 2.00g. It contains calcium carbonate, but no other
substances. It neu...
Questions



Mathematics, 02.09.2019 07:30



Physics, 02.09.2019 07:30


Computers and Technology, 02.09.2019 07:30

English, 02.09.2019 07:30

Mathematics, 02.09.2019 07:30




Mathematics, 02.09.2019 07:30



Health, 02.09.2019 07:30

Mathematics, 02.09.2019 07:30

History, 02.09.2019 07:30