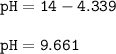Chemistry, 29.11.2020 04:40 devbar3416
Calculate the ph of a solution whose(oh^-) is 4.583*10^-5 mole dm cube (pkw=14)

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 10:50
8) a mixture of he, ne and ar has a pressure of 7.85 atm. if the ne has a mole fraction of 0.47 and 8) ar has a mole fraction of 0.23, what is the pressure of he? a) 4.2 atm b) 3.7 atm c) 5.5 atm d) 2.4 atm e) 1.8 atm
Answers: 1


Chemistry, 22.06.2019 16:30
How many moles of sulfuric acid (h2so4) are needed to react completely with 6.8 moles of lithium hydroxide (lioh)? 2lioh + h2so4 → li2so4 + 2h2o a. 3.4 mol h2so4b. 6.8 mol h2so4 c. 10.2 mol h2so4 d. 13.6 mol h2so4
Answers: 3

Chemistry, 22.06.2019 20:00
The volume of a single vanadium atom is 9.29×10-24 cm3. what is the volume of a vanadium atom in microliters?
Answers: 3
You know the right answer?
Calculate the ph of a solution whose(oh^-) is 4.583*10^-5 mole dm cube (pkw=14)...
Questions




History, 09.01.2020 01:31


Geography, 09.01.2020 01:31


Mathematics, 09.01.2020 01:31


English, 09.01.2020 01:31


Chemistry, 09.01.2020 01:31


Spanish, 09.01.2020 01:31

Mathematics, 09.01.2020 01:31

History, 09.01.2020 01:31




![\tt pOH=-log[4.583\times 10^{-5}]\\\\pOH=5-log~4.583=4.339](/tpl/images/0932/0680/45d8d.png)