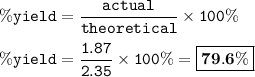Chemistry, 29.11.2020 08:50 zitterkoph
Can someone please help me with this?-- 18 pts!
2C10H11NO4 + 2H2O → C16H10N2O2 + 2C2H4O2 + 4H2O
In this reaction, 1.87 g of indigo (C16H10N2O2) are produced.
If the theoretical yield is 2.35 g of C16H10N2O2, what is the percent yield of indigo?
Round to the nearest decimal.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:40
Chemical kinetics what was the rate of reaction in trial 3? choose the closest answer.
Answers: 3


Chemistry, 22.06.2019 15:00
Areaction is first order with respect to reactant x and second order with respect to reactant y. which statement describes the rate law for this reaction?
Answers: 1

Chemistry, 23.06.2019 01:00
Which of the following is in the lanthanide family? a) uranium b) promethium c) silver d) gold
Answers: 2
You know the right answer?
Can someone please help me with this?-- 18 pts!
2C10H11NO4 + 2H2O → C16H10N2O2 + 2C2H4O2 + 4H2O
Questions

Mathematics, 05.05.2020 15:11

Mathematics, 05.05.2020 15:11

English, 05.05.2020 15:11

English, 05.05.2020 15:11


Mathematics, 05.05.2020 15:11

History, 05.05.2020 15:11

Biology, 05.05.2020 15:11


Mathematics, 05.05.2020 15:11



English, 05.05.2020 15:11

Mathematics, 05.05.2020 15:11

History, 05.05.2020 15:11





Mathematics, 05.05.2020 15:11