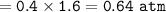Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:00
At 300 mm hg, a gas has a volume of 380 l, what is the volume at standard pressure
Answers: 1

Chemistry, 22.06.2019 11:00
The twister and runaway train are two coasters at the same amusement park. both coasters start at the same height. the coaster for the twister is twice the mass of the coaster for the runaway train. which roller coaster has greater gravitational potential energy at the start of the ride?
Answers: 1


Chemistry, 22.06.2019 14:30
What type(s) of intermolecular forces are expected between ch3ch2cooh molecules? dipole forces, induced dipole forces, hydrogen bonding
Answers: 1
You know the right answer?
In a mixture of 0114 and N2 gases, the mol fraction of C114 is found to be 0.600. The total pressure...
Questions




Biology, 14.05.2020 20:57



Biology, 14.05.2020 20:57





Health, 14.05.2020 20:57

History, 14.05.2020 20:57


Mathematics, 14.05.2020 20:57

History, 14.05.2020 20:57




Mathematics, 14.05.2020 20:57