
Chemistry, 29.11.2020 23:00 chunkymonkey090
Electrolysis of water occurs according to the following equation...
2H20 ->2H2+O2
If electrolysis is performed with 100% efficiency on 100 grams of water. How many grams of hydrogen gas will be produced?
a. 11 grams
b. 50 grams
c. 4 grams
d. 100 grams

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:30
Asample of silver (with work function ? = 4.52 ev) is exposed to an ultraviolet light source (? = 200 nm), which results in the ejection of photoelectrons. what changes will be observed if: silver is replaced with copper (? = 5.10 ev) more photoelectrons ejected no photoelectrons are emitted fewer photoelectrons ejected more energetic photoelectrons (on average) less energetic photoelectrons (on average)
Answers: 3

Chemistry, 22.06.2019 21:30
If you burn 46.6 g of hydrogen and produce 416 g of water, how much oxygen reacted
Answers: 3

Chemistry, 23.06.2019 15:30
Sodium chloride can be made as follows: 2na + cl2 ? 2nacl i calculate the maximum amount of nacl possible if 2.3 g of sodium was reacted with excess chlorine. show all your workings.
Answers: 3

Chemistry, 23.06.2019 18:00
An engineer designing a new type of engine needs a liquid that can be heated and cooled quickly with as little exchange of energy as possible. which property should the engineer primarily look for in the liquid? a. low thermal conductivity b. low specific heat capacity c. high reactivity d. high internal energy e. high density
Answers: 1
You know the right answer?
Electrolysis of water occurs according to the following equation...
2H20 ->2H2+O2
If...
If...
Questions


Mathematics, 06.04.2020 22:42









Social Studies, 06.04.2020 22:42


Mathematics, 06.04.2020 22:42

Mathematics, 06.04.2020 22:42


Mathematics, 06.04.2020 22:42


Mathematics, 06.04.2020 22:43


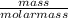
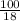 = 5.56mol
= 5.56mol 


