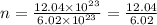16. If you have 12.04 * 1023 molecules of carbon, how many moles of carbon do you
have?...

Chemistry, 30.11.2020 19:20 mihirkantighosh
16. If you have 12.04 * 1023 molecules of carbon, how many moles of carbon do you
have?

Answers: 1


Another question on Chemistry




Chemistry, 22.06.2019 18:00
Chlorophyll a had the molecular formula c55h72mgn4o5 how many atoms are in this molecule
Answers: 2
You know the right answer?
Questions

Biology, 13.09.2019 23:30

Biology, 13.09.2019 23:30


Medicine, 13.09.2019 23:30







Medicine, 13.09.2019 23:30






Biology, 13.09.2019 23:30