
Chemistry, 01.12.2020 03:20 christianfielding336
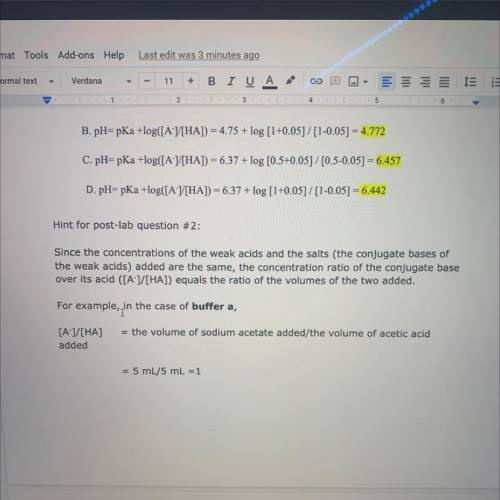
Hint for post-lab question #2:
Since the concentrations of the weak acids and the salts (the conjugate bases of
the weak acids) added are the same, the concentration ratio of the conjugate base
over its acid ([A-]/[HA]) equals the ratio of the volumes of the two added.
For example, in
in the case of buffer a,
= the volume of sodium acetate added/the volume of acetic acid
[A-]/[HA]
added
= 5 mL/5 mL =1

Answers: 3


Another question on Chemistry


Chemistry, 23.06.2019 04:50
The diagin dilutepage 6 of 12a6a5(a)fluorine, chlorine, bromine and iodine are placed in the same group of theperiodic table.state the common name used to describe elements in this group.(i)state the group in which the elements are placed and explain whythey are placed in that group.(ii)which of the above named elements is a solid at roomtemperature and pressure?
Answers: 2

Chemistry, 23.06.2019 11:00
Afraction can be converted to a decimal by dividing the denominator into the numerator. use a calculator. divide to convert the fractions from part a to decimals. then describe the pattern you see in the decimal.
Answers: 3

Chemistry, 23.06.2019 13:40
Match these items with their examples. 1. liquid solution milk 2. solid solution aluminum foil 3. compound soda 4. colloid steel 5. element salt
Answers: 1
You know the right answer?
Hint for post-lab question #2:
Since the concentrations of the weak acids and the salts (the conjug...
Questions

History, 29.01.2020 06:12




Mathematics, 29.01.2020 06:12

Business, 29.01.2020 06:12




Mathematics, 29.01.2020 06:12



Mathematics, 29.01.2020 06:12




Mathematics, 29.01.2020 06:13


Biology, 29.01.2020 06:13

Mathematics, 29.01.2020 06:13



