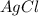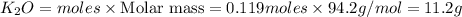Chemistry, 01.12.2020 07:30 ijustneedhelp29
PLEASE HELP
If 9.30 g of potassium reacts with 2.50 g of O2 to form K2O , what is the limiting reagent and what is the theoretical yield of the reaction?
Hint: write the balanced reaction
K - 39.10 g/mol
O - 15.999 g/mol
ANSWER CHOICES:
A.) O2 is limiting, 11.2 g of K2O formed
B.) K is limiting, 14.7 g of K2O formed
C.) K is limiting, 11.2 g of K2O formed
D.) O2 is limiting, 14.7 g of K2O formed
E.) O2 is limiting, 19.2 g of K2O formed

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:30
Acompound has a molar mass of 92.02 grams/mole, and its percent composition is 30.4% nitrogen (n) and 69.6% oxygen (o). what is its molecular formula? a. n2o4 b. no2 c. n2o d. n4o2
Answers: 1

Chemistry, 22.06.2019 12:10
Achemistry student needs to standardize a fresh solution of sodium hydroxide. he carefully weighs out of oxalic acid , a diprotic acid that can be purchased inexpensively in high purity, and dissolves it in of distilled water. the student then titrates the oxalic acid solution with his sodium hydroxide solution. when the titration reaches the equivalence point, the student finds he has used of sodium hydroxide solution.calculate the molarity of the student's sodium hydroxide solution. be sure your answer has the correct number of significant digits.
Answers: 1

Chemistry, 22.06.2019 19:20
Anyone who's in connections academy chemistry b have the factors that affect the rate of a reaction portfolio already done?
Answers: 3

Chemistry, 22.06.2019 23:00
Arectangle has a diagonal 20 inches long that forms angles of 60 and 30 with the sides. find the perimeter of the rectangle. for geometry
Answers: 3
You know the right answer?
PLEASE HELP
If 9.30 g of potassium reacts with 2.50 g of O2 to form K2O , what is the limiting reag...
Questions


Mathematics, 16.10.2020 23:01



Physics, 16.10.2020 23:01



English, 16.10.2020 23:01



Chemistry, 16.10.2020 23:01


Social Studies, 16.10.2020 23:01



Mathematics, 16.10.2020 23:01

History, 16.10.2020 23:01

Biology, 16.10.2020 23:01

Mathematics, 16.10.2020 23:01

History, 16.10.2020 23:01

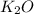 formed
formed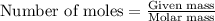
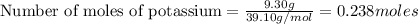
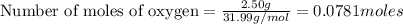
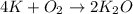
 require 1 mole of
require 1 mole of 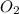
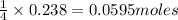 of
of 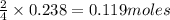 of
of