PLEASE HELP
give the electron configuration of vanadium (V), atomic number 23
Give the...

PLEASE HELP
give the electron configuration of vanadium (V), atomic number 23
Give the noble gas configuration of vanadium (V), atomic number 23
List the energy levels for the orbital configuration of vanadium (V), atomic number 23
How does the atomic radius change going down and across the periodic table?
How does the first ionization energy change going down and across the periodic table?
How does electronegativity change going down and across the periodic table?
How does the radius of a positive and negative ion compared to a neutral atom?
Match: A. Ionic bond B. Covalent bond C. Metallic bond __sharing of electrons __freely moving electrons __transfer electrons
Describe the dipole dipole force
Describe hydrogen bonding
Describe the Van der waals forces
Imagine you need to take a medicine that the doctor has prescribed for you. Explain why scientist who developed that medicine would need to know whether or not the compound in that medicine is polar. How might a polar medicine behave differently within your body than a non-polar medicine word? (Answer in 1 to 2 paragraphs)
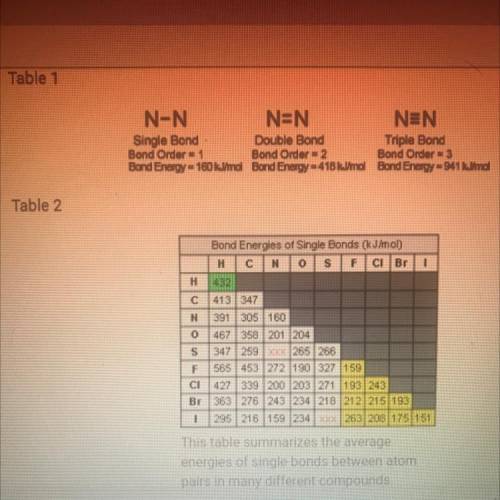
According to table 2, which is the strongest bond? Which is the week is fine? Based on what you know about the atomic radii and electronegativity of the elements involved in the bonds, why do you think these to have the most extreme bond-energy values?
How are the bond energies of each bond listed in table two determined?
Why do you think there aren’t bond energy values given in table 2 for N-S and S-I?
Based on tables one and two how would you describe the trend in bond strength of single double and triple bonds?
Based on table 2, how would you describe the trend in strength of bonds formed by the elements carbon nitrogen and oxygen? Would you describe this trend as a periodic trend? Why or why not?
What is the VSEPR theory?
How does electron repulsion determine molecular shape?
How do lone electron pairs affect molecular shape?
Draw the Lewis structure for the Se and 2 H atoms
Draw the Lewis structure for the SeH(2) molecule
What shape would SeH(2) have? Draw the molecule
Identify each of the following as a covalent compound, or ionic compound. Then provide either the formula for compounds identified by name or the name for those identified by formula:
Li(2)O
Dibitrogen trioxide
PCl(3)
Manganese(|||) oxide
Calcium bromide
THANK YOU FOR YOUR HELP <3

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Which best describes why nh4+ can form an ionic bond with ci-?
Answers: 1

Chemistry, 22.06.2019 05:50
In an exothermic reaction the bonding energy of the product is: less than the reactants same as the reactants greater than the reactants dependent upon the presence of a catalyst
Answers: 1

Chemistry, 22.06.2019 09:00
Suppose you have designed a new thermometer called the x thermometer. on the x scale the boiling point of water is 129 ? x and the freezing point of water is 13 ? x. part a at what temperature are the readings on the fahrenheit and x thermometers the same?
Answers: 1

Chemistry, 22.06.2019 18:00
Hydrogenation reactions, in which h2 and an "unsaturated" organic compound combine, are used in the food, fuel, and polymer industries. in the simplest case, ethene (c2h4) and h2 form ethane (c2h6). if 140 kj is given off per mole of c2h4 reacting, how much heat (in mj) is released when 12 kg of c2h6 forms?
Answers: 2
You know the right answer?
Questions

Mathematics, 20.08.2020 09:01

History, 20.08.2020 09:01

Mathematics, 20.08.2020 09:01

Mathematics, 20.08.2020 09:01




Mathematics, 20.08.2020 09:01




Physics, 20.08.2020 09:01

Mathematics, 20.08.2020 09:01

Mathematics, 20.08.2020 09:01

Mathematics, 20.08.2020 09:01





Mathematics, 20.08.2020 09:01



