
Chemistry, 01.12.2020 18:40 lafayette7727
45 POINTS
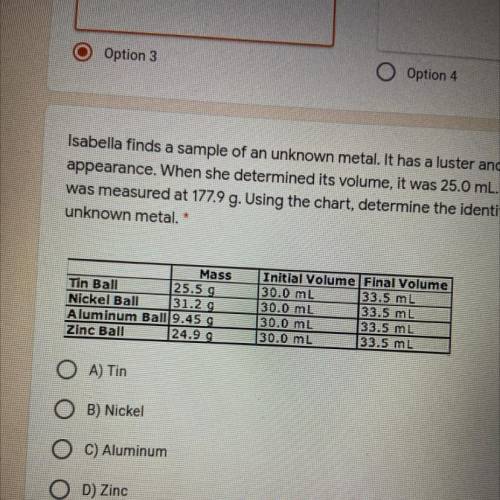
Isabella finds a sample of an unknown metal. It has a luster and is silvery in 2 points
appearance. When she determined its volume, it was 25.0 ml. Its mass
was measured at 177.9 g. Using the chart, determine the identity of the
unknown metal. *
Mass
Tin Ball
25.5 9
Nickel Ball 31.29
Aluminum Ball 9.459
Zinc Ball
24.99
Initial Volume Final Volume
30.0 mL 33.5 mL
30.0 mL 33.5 mL
30.0 mL 33.5 mL
30.0 mL
33.5 ml
A) Tin
B) Nickel
C) Aluminum
D) Zinc

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 16:00
Silica, sio2, is formed on silicon as an electrically insulating layer for microelectronic devices. silica is formed when silicon is exposed to o2 gas at an elevated temperature. at 900˚c, it takes 90 minutes for the oxygen to diffuse from the surface to form a 0.06 micron (0.06 x 10-6 m) thick layer of sio2 on
Answers: 1

Chemistry, 21.06.2019 17:40
What are the resulting coefficients when you balance the chemical equation for the combustion of ethane, c2h6? in this reaction, ethane is burned in the presence of oxygen (o2) to form carbon dioxide (co2) and water (h2o). (g)+(g)→(g)+(g)
Answers: 1

Chemistry, 21.06.2019 22:00
Which describes interactions between substances and stomata during photosynthesis? check all that apply. oxygen enters stomata. oxygen is released through stomata. carbon dioxide enters stomata. carbon dioxide is released through stomata. hydrogen enters stomata. hydrogen is released through stomata.
Answers: 1

You know the right answer?
45 POINTS
Isabella finds a sample of an unknown metal. It has a luster and is silvery in 2 points
Questions




Mathematics, 04.12.2020 16:30

Social Studies, 04.12.2020 16:30


Mathematics, 04.12.2020 16:30

Biology, 04.12.2020 16:30

Computers and Technology, 04.12.2020 16:30

Biology, 04.12.2020 16:30

English, 04.12.2020 16:30





Computers and Technology, 04.12.2020 16:30







