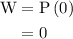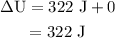Chemistry, 23.12.2019 04:31 pearlkissp1bzl8
Calculate the change in internal energy of the following system: a 100.0-g bar of gold is heated from 25 ∘c to 50 ∘c during which it absorbs 322 j of heat. assume the volume of the gold bar remains constant.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:00
Identify a strong intermolecular force of attraction between an alcohol
Answers: 1

Chemistry, 22.06.2019 09:40
Apiece of copper has a temperature of 75.6 0c. when the metal is placed in 100.0 grams of water at 19.1 0c, the temperature rises by 5.5 0c. what is the mass of the metal?
Answers: 1

Chemistry, 22.06.2019 12:00
Give the set of reactants (including an alkyl halide and a nucleophile) that could be used to synthesize the following ether: draw the molecules on the canvas by choosing buttons from the tools (for bonds and charges), atoms, and templates toolbars, including charges where needed. ch3ch2och2ch2chch3 | ch3
Answers: 1

Chemistry, 22.06.2019 20:30
What is a difference between a mixture of elements and a mixture of compounds
Answers: 1
You know the right answer?
Calculate the change in internal energy of the following system: a 100.0-g bar of gold is heated fr...
Questions

Chemistry, 27.05.2020 02:57




Mathematics, 27.05.2020 02:57

Mathematics, 27.05.2020 02:57

Mathematics, 27.05.2020 02:57

SAT, 27.05.2020 02:57

Mathematics, 27.05.2020 02:57



History, 27.05.2020 02:57

Mathematics, 27.05.2020 02:57

Mathematics, 27.05.2020 02:57

Mathematics, 27.05.2020 02:57





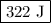
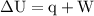 …… (1)
…… (1)
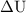 is the change in internal energy of the system.
is the change in internal energy of the system.
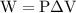 …… (2)
…… (2)
 is the change in the volume of the system.
is the change in the volume of the system.