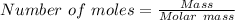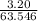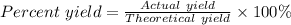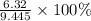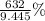Chemistry, 02.12.2020 01:00 angelica7773
Suppose 3.20 g of copper are reacted with excess nitric acid according to the given equation, and 6.32 g Cu(NO3)2 product are obtained.
Cu(s) + 4 HNO3 (aq) --> Cu(NO3)2 (aq) + 2 NO2 (g) + 2 H2O(l)
What is the theoretical yield of Cu(NO3)2? In g
What is the percent yield of Cu(NO3)2? In %

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:30
1. explain hydrogen peroxide, h 2 o 2 properties and decomposition reaction. 2. describe how each of the following natural cycles plays a part in earth’s climate system. (a) the water cycle (b) the carbon cycle
Answers: 1

Chemistry, 22.06.2019 15:30
Draw the lewis dot structure for each of the following polyatomic ions
Answers: 1

Chemistry, 22.06.2019 19:30
How might this scientific phenomena be explained? a paper clip floats on water.
Answers: 1

Chemistry, 22.06.2019 21:20
If a simple machine aduces the strength of a force, what must be increased? the speed of the input force the work the simple machine performs the size of the simple machine the distance over which the force is applied
Answers: 1
You know the right answer?
Suppose 3.20 g of copper are reacted with excess nitric acid according to the given equation, and 6....
Questions

Mathematics, 20.07.2021 21:20


Mathematics, 20.07.2021 21:20


Chemistry, 20.07.2021 21:20

Mathematics, 20.07.2021 21:20

Mathematics, 20.07.2021 21:20

Mathematics, 20.07.2021 21:20



Mathematics, 20.07.2021 21:20

Mathematics, 20.07.2021 21:20

Mathematics, 20.07.2021 21:20

Mathematics, 20.07.2021 21:20


History, 20.07.2021 21:20


Mathematics, 20.07.2021 21:20