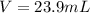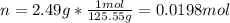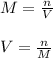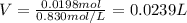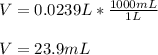Chemistry, 02.12.2020 14:00 isabelperez063
1. If 2.49 g of CuNO3 is dissolved in water to make a 0.830 M solution, what is the volume of the solution in milliliters?
2. How many moles of NaOH are present in 13.5 mL of 0.170 M NaOH?
3. Calculate the molarity of 0.650 mol of Na2S in 1.15 L of solution.
4. A student in lab needs to make a solution that is 7.00% by mass NaCl. If 145 g of NaCl is available, what mass of solution can be prepared?
5. Calculate the molarity of 24.1 g of MgS in 777 mL of solution.
6. A solution made by adding 16.3mL of methyl alcohol to enough water to give 541
mL of solution.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 12:00
Ageochemist examines a piece of metal that he found in the soil. he performs tests to identify the metal from its density, electrical conductivity, and melting point. which statement best describes his investigation? a. he is determining physical properties that are sufficient to identify the metal.b. he is determining chemical properties that are sufficient to identify the metal.c. he is determining physical properties that are insufficient to identify the metal.d. he is determining chemical properties that are insufficient to identify the metal.
Answers: 3

Chemistry, 22.06.2019 12:50
What is the chemical name of the compound na2co3? use the list of polyatomic ions and the periodic table to you answer. a. sodium carbon oxide b. sodium carbonate c. sodium(ll) carbonate d. sodium oxalate
Answers: 1

Chemistry, 22.06.2019 23:00
What is a substance? a. a physical property of matter b. a chemical property of matter c. an element or compound that cannot be physically separated d. characteristics used to tell the difference between mixtures
Answers: 1

You know the right answer?
1. If 2.49 g of CuNO3 is dissolved in water to make a 0.830 M solution, what is the volume of the so...
Questions


Mathematics, 08.09.2020 04:01

Geography, 08.09.2020 04:01

Mathematics, 08.09.2020 04:01








Law, 08.09.2020 04:01



Social Studies, 08.09.2020 04:01