Chemistry, 02.12.2020 18:30 MrKrinkle77
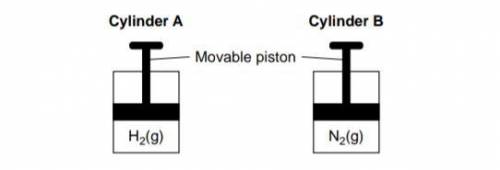
Cylinder A and Cylinder B are sealed, rigid cylinders with movable pistons. Each cylinder contains 500. milliliters of a gas sample at 101.3 kPa and 298 K. Cylinder A contains H2 and cylinder B contains N2. Explain, in terms of collisions between gas molecules and the walls of the container, why pushing the movable piston farther into cylinder B at constant temperature would increase the pressure of the N2 gas.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:00
The p sub shell can hold up to 8 electrons in an atom. true or false?
Answers: 1

Chemistry, 22.06.2019 08:40
What is the value of keq for the reaction expressed in scientific notation?
Answers: 1

Chemistry, 22.06.2019 09:00
Which process does not require the presence of a physical substance in order to transfer heat? air in the atmosphere is heated by the ground. this warm air then rises, and cooler air falls. this is an example of what type of process? how is conduction different from radiation?
Answers: 1

Chemistry, 22.06.2019 20:00
How are the terms group and period used on the periodic table
Answers: 1
You know the right answer?
Cylinder A and Cylinder B are sealed, rigid cylinders with movable pistons. Each cylinder contains 5...
Questions

English, 26.02.2021 20:50


Mathematics, 26.02.2021 20:50

Mathematics, 26.02.2021 20:50

English, 26.02.2021 20:50



Mathematics, 26.02.2021 20:50

Mathematics, 26.02.2021 20:50





Mathematics, 26.02.2021 20:50




Mathematics, 26.02.2021 20:50

Mathematics, 26.02.2021 20:50