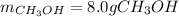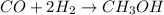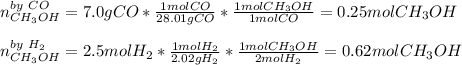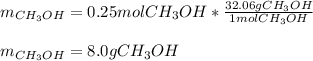A synthesis reaction takes place when carbon monoxide (CO) and hydrogen gas (H2) react to form methanol (CH3OH). How many grams of methanol are produced when 7.0 grams of carbon monoxide reacts with 2.5 grams of hydrogen gas? (5 points) 7.0 grams Ob 8.0 grams 15 grams d 20 grams

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Agood hypothesis includes which of the following? a: prediction b: data c: uncertainty d: conclusion
Answers: 1


Chemistry, 22.06.2019 20:30
Draw a line graph showing the relationship between temperature in kelvin as a function of kinetic energy.
Answers: 3

You know the right answer?
A synthesis reaction takes place when carbon monoxide (CO) and hydrogen gas (H2) react to form metha...
Questions





Mathematics, 19.04.2020 12:34

Geography, 19.04.2020 12:34

Health, 19.04.2020 12:34


World Languages, 19.04.2020 12:34

Health, 19.04.2020 12:35

Mathematics, 19.04.2020 12:35

Mathematics, 19.04.2020 12:36

Mathematics, 19.04.2020 12:36

Spanish, 19.04.2020 12:37

Social Studies, 19.04.2020 12:37

History, 19.04.2020 12:37

Mathematics, 19.04.2020 12:37



Mathematics, 19.04.2020 12:52