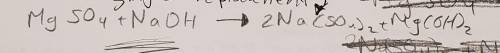Chemistry, 02.12.2020 21:30 irvinbhangal2
3. Magnesium sulfate is added to sodium hydroxide to produce sodium sulfate and magnesium hydroxide (10 points)
a. Write the skeletal equation and Balance the chemical equation describing the reaction above.
b. What kind of reaction is this?
c. Rewrite the balanced chemical equation, adding state symbols for all compounds in the reaction.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:30
Acompound has a molar mass of 92.02 grams/mole, and its percent composition is 30.4% nitrogen (n) and 69.6% oxygen (o). what is its molecular formula? a. n2o4 b. no2 c. n2o d. n4o2
Answers: 1

Chemistry, 23.06.2019 00:50
What is the enthalpy of combustion (per mole) of c4h10 (g)? –2,657.5 kj/mol –5315.0 kj/mol –509.7 kj/mol –254.8 kj/mol
Answers: 1

Chemistry, 23.06.2019 01:00
Which elements are found in glucose, the product of photosynthesis? a. carbon, hydrogen, and oxygen b. carbon and hydrogen c. carbon, nitrogen, and oxygen d. hydrogen, nitrogen, and carbon
Answers: 2

Chemistry, 23.06.2019 02:00
What causes the appearance of lines in a emission spectrum
Answers: 1
You know the right answer?
3. Magnesium sulfate is added to sodium hydroxide to produce sodium sulfate and magnesium hydroxide...
Questions





Mathematics, 17.04.2020 22:17

Mathematics, 17.04.2020 22:17


Mathematics, 17.04.2020 22:17

Mathematics, 17.04.2020 22:17

History, 17.04.2020 22:17


Mathematics, 17.04.2020 22:17

Social Studies, 17.04.2020 22:17


Mathematics, 17.04.2020 22:17

History, 17.04.2020 22:17

Mathematics, 17.04.2020 22:17



Mathematics, 17.04.2020 22:18