
Chemistry, 03.01.2020 02:31 audreymarie2940
Calculate the hydrogen-ion concentration [h+] for the aqueous solution in which [oh–] is 1 x 10–11 mol/l. is this solution acidic, basic, or neutral? show all work.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Why is soap used to remove grease? a. its nonpolar end dissolves the grease. b. it makes the water bond with the grease. c. it chemically bonds with the grease. d. its polar end dissolves the grease.correct answer for apex - a, its nonpolar end dissolves the grease.
Answers: 1


Chemistry, 22.06.2019 15:30
The gulf stream is a warm water current that flows away from the equator to northern europe. witch of these does it cause. a. crashes of warm and cool water in the ocean b.colder climates near the equator c.large waves on the cost of europe d.warm climates in northern europe
Answers: 1

Chemistry, 22.06.2019 19:40
What type of electromagnetic waves does the human eye see as the colors red blue or green a visible light waves b radio waves c infrared waves d microwaves
Answers: 1
You know the right answer?
Calculate the hydrogen-ion concentration [h+] for the aqueous solution in which [oh–] is 1 x 10–11 m...
Questions




Mathematics, 20.06.2020 21:57


Mathematics, 20.06.2020 21:57



Mathematics, 20.06.2020 21:57








Mathematics, 20.06.2020 21:57

History, 20.06.2020 21:57

Mathematics, 20.06.2020 21:57

![[H+][OH-]= Kw = 1.0 x 10^-14](/tpl/images/0440/7437/9a04c.png)
![[H+]= Kw/ [OH-]= 1.0x 10^-14](/tpl/images/0440/7437/e7cfa.png)
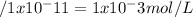
![pH = - log [H+]= - log 1 x 10^-3 = 3 \ \textless \ 7](/tpl/images/0440/7437/22063.png)


