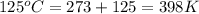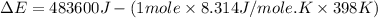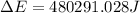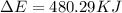Chemistry, 31.08.2019 00:00 nathaniel12
Consider the reaction: 2 h2o (> 2 h2 (g) + o2 (g). δh=483.6 kj/mol. if 2 moles of h2o (g) are converted h2(g) and o2(g) against a pressure of 1 atm at 125 degrees celcius what is δe of reaction?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:30
What are scientists who study fossils called? ( a ) astronomers. ( b ) biologists. ( c ) geologists. ( d ) paleontologists.
Answers: 2


Chemistry, 22.06.2019 12:00
Which statement best explains the relationship between an area is geography and the temperature of its surface water
Answers: 1

Chemistry, 22.06.2019 12:30
In france, grapes are 1.95 euros per kilogram. what is the cost of grapes, in dollars per pound, if the exchange rate is 1.14 dollars/euro? (2.6)
Answers: 3
You know the right answer?
Consider the reaction: 2 h2o (> 2 h2 (g) + o2 (g). δh=483.6 kj/mol. if 2 moles of h2o (g) are co...
Questions

Mathematics, 02.10.2020 09:01

Mathematics, 02.10.2020 09:01


Arts, 02.10.2020 09:01



Advanced Placement (AP), 02.10.2020 09:01



Mathematics, 02.10.2020 09:01

Mathematics, 02.10.2020 09:01




Chemistry, 02.10.2020 09:01

Mathematics, 02.10.2020 09:01


Law, 02.10.2020 09:01


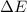 of the reaction is, 480.29 KJ.
of the reaction is, 480.29 KJ.
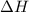 = enthalpy of the reaction = 483.6 KJ/mole = 483600 J
= enthalpy of the reaction = 483.6 KJ/mole = 483600 J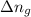 = change in the moles of the reaction = Moles of product - Moles of reactant = 3 - 2 = 1 mole
= change in the moles of the reaction = Moles of product - Moles of reactant = 3 - 2 = 1 mole