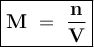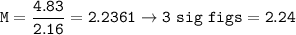Answers: 2


Another question on Chemistry


Chemistry, 23.06.2019 00:00
What is the approximate mass of 25 cm3 of silver, if the density is 10.5 g/cm3? a. 0.42 g b. 2.4 g c. 42 g d. 260 g
Answers: 1

Chemistry, 23.06.2019 01:00
Substance 33°f 100°f peanut oil solid liquid margarine solid liquid chocolate chips solid liquid which conclusion fits the data in the table? a. heat chemically changes chocolate and margarine. b. all solids become liquid at 100°f. c. removing heat from a substance it to melt. d. matter may change shape when it is heated.
Answers: 1

Chemistry, 23.06.2019 04:20
Which activity describes an application of topographic maps? check all that apply. recreation, such as camping and hiking engineering, such as the construction of roads and buildings science, such as mapping stars in the sky business, such as analyzing population centers science, such as analyzing surface features
Answers: 1
You know the right answer?
Determine the molarity of a solution formed by dissolving 4.83 moles of NaCl (M=58.44 g/mol) in enou...
Questions

Mathematics, 12.12.2020 16:50



Arts, 12.12.2020 16:50


Mathematics, 12.12.2020 16:50



Mathematics, 12.12.2020 16:50










Computers and Technology, 12.12.2020 16:50

Mathematics, 12.12.2020 16:50