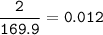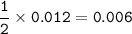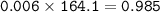Chemistry, 03.12.2020 14:00 guzmanbrandon3259
The following reaction shows calcium chloride reacting with silver nitrate.
CaCl2 + 2AgNO3 → 2AgCl + Ca(NO3)2
How many grams of Ca(NO3)2 are produced from 2,000.0 grams of AgNO3?
(Molar mass of Ca = 40.1 g/mol, Cl = 35.5 g/mol, O = 16.0 g/mol, Ag = 107.9 g/mol, N = 14.0 g/mol)
(Show your calculations for full credit. Correct answers will only receive partial credit.)

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:00
Different isotopes indicate that an element will have different numbers of
Answers: 2

Chemistry, 22.06.2019 22:00
11) burning your hand when accidentally touching a hot plate is an example of which heat transfer? a. conduction b. convection c. radiation d. none of these
Answers: 2

Chemistry, 23.06.2019 02:30
Calculate the ph at the equivalence point for the titration of a solution containing 150.0 mg of ethylamine (c2h5nh2) with 0.1000 m hcl solution. the volume of the solution at the equivalence point is 250.0 ml. kb forethylamine is 4.7 × 10−4 .
Answers: 2

Chemistry, 23.06.2019 06:00
How does a coronal mass ejection (cme) affect the solar wind? a cme adds more particles to the solar wind, intensifying it. a cme blocks the solar wind, causing it to fade. a cme does not affect the solar wind but it does affect auroras. a cme increases the amount of energy in the solar wind.
Answers: 2
You know the right answer?
The following reaction shows calcium chloride reacting with silver nitrate.
CaCl2 + 2AgNO3 → 2AgCl...
Questions

Mathematics, 04.03.2021 18:20

History, 04.03.2021 18:20


Mathematics, 04.03.2021 18:20


Mathematics, 04.03.2021 18:20

Mathematics, 04.03.2021 18:20

Mathematics, 04.03.2021 18:20

Mathematics, 04.03.2021 18:20

Social Studies, 04.03.2021 18:20


Physics, 04.03.2021 18:20

Social Studies, 04.03.2021 18:20




Mathematics, 04.03.2021 18:20