
Chemistry, 03.12.2020 14:20 babyduckies37
25.0cm3 of s saturated potassium hydroxide is neutralized by 35.0cm3 of hydrogen chloride acid of concentration 0.75 mol/dm3. Calculate the concentration of potassium hydroxide solution. Please help, will give brainliest, unhelpful answers will get reported.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:00
Drag each number to the correct location on the equation. each number can be used more than once, but not all numbers will be used. balance the equation with the coefficients. 2 3 4 5 kclo3 -> kcl + o2
Answers: 1

Chemistry, 21.06.2019 20:30
Hannah is writing a report on how albedo affects the global climate. she’s proofreading her passage for any factual errors. which sentence must hannah correct before submitting her report? earth receives energy from the sun. this energy drives many of the processes on earth, including its climate. some part of this energy is reflected by earth’s surface. we use the term albedo to describe the reflected energy. albedo of an object is the ratio of the reflected radiation to the total radiation reaching the object. a value of 0 means no energy is absorbed by the object, whereas a value of 1 means that all of the energy is absorbed. in this way, the albedo of an object can influence earth’s atmospheric temperature.
Answers: 1

Chemistry, 22.06.2019 04:00
What three natural resources are found in the great lakes region
Answers: 2

Chemistry, 22.06.2019 12:30
Clyde and marilyn are riding a roller coaster. during which section(s) of the track is their potential energy converted to kinetic energy? a. from point b to point c only b. from point b to point d only c. from point a to point b only d. from point a to point b and from point c to point d
Answers: 1
You know the right answer?
25.0cm3 of s saturated potassium hydroxide is neutralized by 35.0cm3 of hydrogen chloride acid of co...
Questions


English, 28.08.2019 14:20

Biology, 28.08.2019 14:20


Mathematics, 28.08.2019 14:20

English, 28.08.2019 14:20

Mathematics, 28.08.2019 14:20


Computers and Technology, 28.08.2019 14:20

Mathematics, 28.08.2019 14:20



English, 28.08.2019 14:20

Mathematics, 28.08.2019 14:20


History, 28.08.2019 14:20


Mathematics, 28.08.2019 14:20


Mathematics, 28.08.2019 14:20

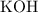 solution: approximately
solution: approximately 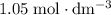 .
. 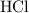 solution is in the unit
solution is in the unit 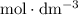 . However, the unit of the two volumes is
. However, the unit of the two volumes is 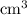 . Convert the unit of the two volumes to
. Convert the unit of the two volumes to 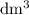 to match the unit of concentration.
to match the unit of concentration.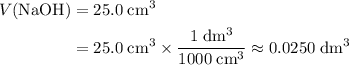 .
.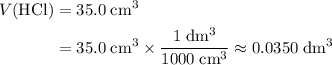 .
.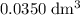 of
of 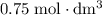
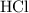 solution:
solution: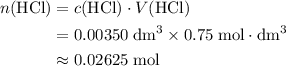 .
.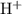 .
.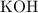 formula unit would react with up to one
formula unit would react with up to one 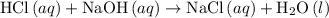 .
.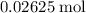 of
of 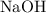 formula units. That is:
formula units. That is: 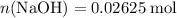 .
.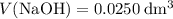 and
and 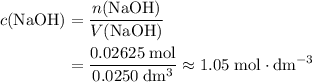 .
.

