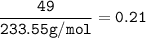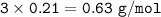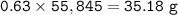Chemistry, 03.12.2020 14:20 bgallman153p71edg
In if the reaction below 49 grams of Fe3O4 produces a 78.25% yield of Fe. How many grams are produced? Fe3O4 + 4H2 = 3Fe + 4H2O Imagine the = is an arrow

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:30
How much energy is made when a pice of wood burns. how do you know
Answers: 2

Chemistry, 22.06.2019 04:30
How do i complete this electrolysis of water lab? i’m at home, so i don’t have the materials, and the lab didn’t properly work and was incomplete at school.
Answers: 1

Chemistry, 22.06.2019 08:30
For each of the compounds below, show that the charges on the ions add up to zero. a. kbr b. cao c. li(2)o d. cacl(2) e. alcl(3)
Answers: 2

Chemistry, 22.06.2019 19:00
Mercury metal is poured into a graduated cylinder that holds exactly 22.5 ml the mercury used to fill the cylinder mass in 306.0 g from this information calculate the density of mercury
Answers: 2
You know the right answer?
In if the reaction below 49 grams of Fe3O4 produces a 78.25% yield of Fe. How many grams are produce...
Questions

Computers and Technology, 09.12.2021 03:50


Mathematics, 09.12.2021 03:50

Geography, 09.12.2021 03:50




Mathematics, 09.12.2021 03:50



Mathematics, 09.12.2021 03:50


Health, 09.12.2021 03:50


Spanish, 09.12.2021 03:50

Mathematics, 09.12.2021 03:50




Mathematics, 09.12.2021 03:50