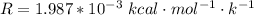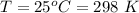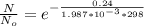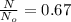Chemistry, 03.12.2020 17:20 eggoysters
1. The thermal interconversion of the axial and equatorial substitutents of the chair conformation of cyclohexane is extremely slow because the two forms are separated by a relatively high activation energy barrier, 10.5 kcal/mol (3672 cm−1). With CN substituents, the equatorial form is only 0.24 kcal/mol (84 cm−1) lower in energy than the axial form. Show how you would determine the ratio between the concentrations of the equatorial and axial forms using the Boltzmann distribution.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 03:50
What is the temperature of one mole of helium gas at stp?
Answers: 3

Chemistry, 22.06.2019 17:00
Astable electron arrangement for an atom is one that does not easily change. how is this arrangement arrived at? a. valence electrons are transferred or shared to create a full outer shell of electrons. b. valence electrons are discarded into space to create a full outer shell of electrons. c. protons (positive charge) pair with valence electrons (negative charge) to create a strong bond. d. outer shells with valence electrons are transferred or shared.
Answers: 2

Chemistry, 23.06.2019 03:50
How many moles of potassium are needed to react completely with 12.8 moles of magnessium bromide?
Answers: 2
You know the right answer?
1. The thermal interconversion of the axial and equatorial substitutents of the chair conformation o...
Questions

Mathematics, 22.04.2021 02:30

History, 22.04.2021 02:30

Law, 22.04.2021 02:30

Spanish, 22.04.2021 02:30

Biology, 22.04.2021 02:30

Mathematics, 22.04.2021 02:30


Mathematics, 22.04.2021 02:30


Chemistry, 22.04.2021 02:30

Mathematics, 22.04.2021 02:30

History, 22.04.2021 02:30

Mathematics, 22.04.2021 02:30




Mathematics, 22.04.2021 02:30



Mathematics, 22.04.2021 02:30

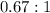
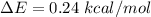
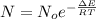
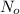 is the number of molecule in equatorial form and N is the number of the molecules in the axial form.
is the number of molecule in equatorial form and N is the number of the molecules in the axial form.