
Chemistry, 03.12.2020 18:10 fernandaElizondo
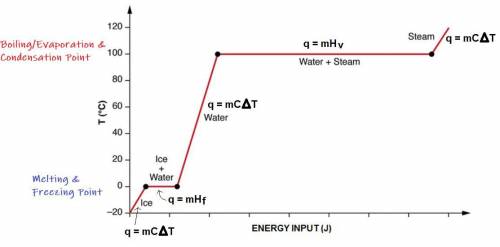
A 200 g sample of water at 60.0 degrees Celsius is heated to water vapor at 140.0 degrees Celsius. Expected Answer = 501,440 J Before trying to solve this problem, explain:
what is happening to the water from 60.0 degrees Celsius to 100.0 degrees Celsius?
what happens at 100.0 degrees Celsius?
what happens from 100.0 degrees Celsius to 140.0 degrees Celsius?
Then solve the full problem, showing work & units.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 16:30
Acetic acid, hc2h3o2, dissolves and completely dissociates in water and a solvation sphere of water molecules forms around the ions. this solute-solvent interaction
Answers: 1

Chemistry, 22.06.2019 03:50
What is the temperature of one mole of helium gas at stp?
Answers: 3

Chemistry, 22.06.2019 14:20
Which of the following are sources of revenue for media companies? a. direct sales to producers b.advertising and subscriptions c. online purchase d. capital investments
Answers: 1

Chemistry, 22.06.2019 16:50
Ajet plane is speeding down the runway during takeoff. air resistance is not negligible. identify the forces on the jet.
Answers: 3
You know the right answer?
A 200 g sample of water at 60.0 degrees Celsius is heated to water vapor at 140.0 degrees Celsius. E...
Questions

History, 01.03.2021 17:20

Mathematics, 01.03.2021 17:20

Mathematics, 01.03.2021 17:20


Chemistry, 01.03.2021 17:20

English, 01.03.2021 17:20


English, 01.03.2021 17:20

Mathematics, 01.03.2021 17:20


Engineering, 01.03.2021 17:20

English, 01.03.2021 17:20




Mathematics, 01.03.2021 17:20

Biology, 01.03.2021 17:20

Mathematics, 01.03.2021 17:20


Mathematics, 01.03.2021 17:20



