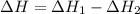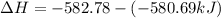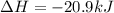Chemistry, 03.12.2020 20:10 haileywebb8
The phase change between white tin and gray tin is difficult to observe directly. Both substances can be burned, however. From these equations, calculate AHⓇ for the conversion of gray tin into white tin:
Sn(s, white) + O2(g) + SnO2(g) AH = -580.69 kJ
Sn(s, gray) + O2(g) + SnO2(g) AH° = -582.78 kJ
AH = kJ

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:10
The peak wavelength for the blackbody curve of a star is in the uv range. assuming the radiation from this star can reach earth, would you be able to see it?
Answers: 2


Chemistry, 22.06.2019 14:30
What type(s) of intermolecular forces are expected between ch3ch2cooh molecules? dipole forces, induced dipole forces, hydrogen bonding
Answers: 1

Chemistry, 22.06.2019 14:40
Choose an equation that represents an enzyme-catalyzed reaction. (a) enzyme + substrate → enzyme-substrate complex (b) enzyme + substrate ←−→ enzyme + products (c) enzyme + substrate ←−→ enzyme-substrate complex → enzyme + products (d) enzyme + substrate ←−→ enzyme-substrate complex → enzyme-substrate complex + products
Answers: 2
You know the right answer?
The phase change between white tin and gray tin is difficult to observe directly. Both substances ca...
Questions

Arts, 14.12.2020 23:10




Mathematics, 14.12.2020 23:10



Social Studies, 14.12.2020 23:10

Mathematics, 14.12.2020 23:10

English, 14.12.2020 23:10

Mathematics, 14.12.2020 23:10








English, 14.12.2020 23:10

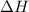 for the conversion of gray tin to white tin is -20.9 kJ
for the conversion of gray tin to white tin is -20.9 kJ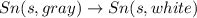
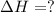
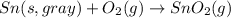
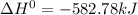 (1)
(1)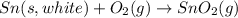
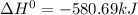 (2)
(2)