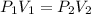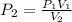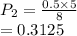Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:30
The best solution for preventing harm to people and pets from severe hurricanes involves determining and warning residents about what
Answers: 1

Chemistry, 22.06.2019 21:00
Once similarity and one difference between a mixture of elements and a mixture of compounds
Answers: 3

Chemistry, 22.06.2019 22:00
Ill give u brainliest pls how is mass of carbon conserved during cellular respiration
Answers: 1

Chemistry, 22.06.2019 23:30
The density of the solid phase of a substance is 0.90 g/cm3 and the density of the liquid phase is 1.0 g/cm3. a large increase in pressure will a. lower the freezing point b. raise the freezing point c. lower the boiling point d. raise the triple point e. lower the triple point
Answers: 1
You know the right answer?
The volume of a gas with an initial pressure of 380 mmHg atm increases from 5.0 L to 8.0 L. What is...
Questions


Biology, 25.09.2019 20:30

History, 25.09.2019 20:30


Physics, 25.09.2019 20:30

Mathematics, 25.09.2019 20:30



Chemistry, 25.09.2019 20:30


Mathematics, 25.09.2019 20:30


Mathematics, 25.09.2019 20:30




Mathematics, 25.09.2019 20:30