
Modeling Energy Changes Student Guide on Edge
Step 2: Write and Balance the Chemical Equation.
a) Read the word equation: "Propane gas plus oxygen gas produces _."
b) Convert the word equation to a chemical equation and complete the reaction. Be sure to balance the chemical equation.
c) Record the balanced chemical equation on the Student Worksheet.
Step 3: Determine the amount of energy change in the reaction.
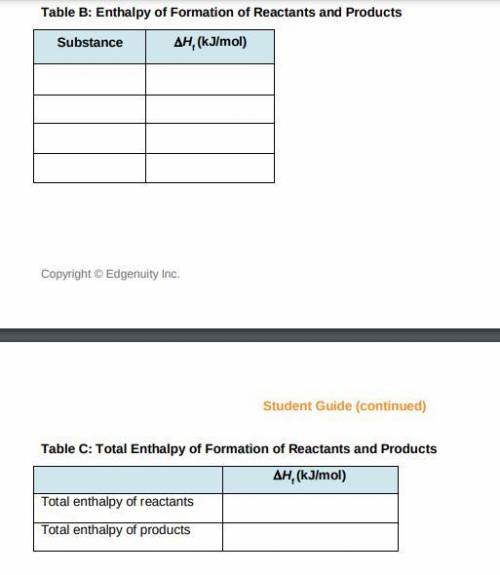
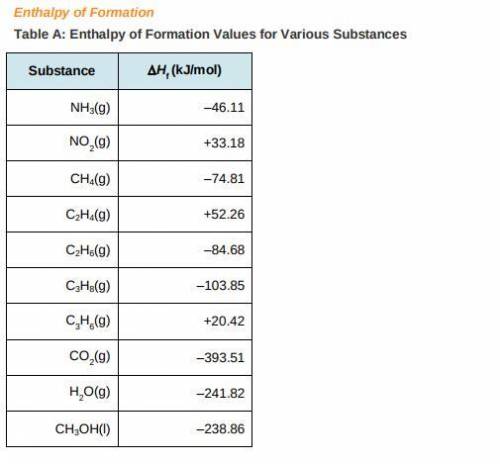
a) Use the table of enthalpy values (Table A) provided in the Student Worksheet to locate the enthalpy of formation (DeltaHt) for each reactant and each product. Record these values along with the reactants and products in Table B of the Student Worksheet.
b) Determine the total enthalpy of the reactants and the total enthalpy of the products Record these values in Table C of the Student Worksheet.
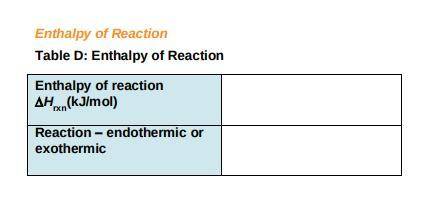
c) Use the following formula to find the net change in enthalpy for the reaction and to determine whether the reaction is endothermic is endothermic or exothermic.
DeltaHrxn= E (Delta Hf, products)- E (DeltaHf, ractants)
Record your answers in Table D.
Step 4: Model the energy change in the reaction.
a) Create an energy graph that illustrates the energy change in the reaction.
b)Construct your graph on a blank sheet of paper. Be sure to label the axes, provide a title, and identify the reactants and product on the graph.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 11:00
Which statement is true about hcl? (5 points) select one: a. it is a salt because it increases the concentration of metallic ions. b. it is a salt because it is formed by the reaction of an acid and a base. c. it is an acid because it increases the concentration of hydroxyl ions. d. it is an acid because it increases the concentration of hydronium ions.
Answers: 1

Chemistry, 22.06.2019 19:20
15. which of the following is not human-caused groundwater pollution? a. water in an aquifer dissolves elements such as arsenic and mercury from surrounding rock. b. water in an aquifer is contaminated by leachate that seeps into the ground from a landfill. c. water in an aquifer becomes polluted with chemicals used in hydraulic fracturing, or fracking. d. water in an aquifer absorbs harmful bacteria from the drainage field of a septic tank.
Answers: 1

Chemistry, 22.06.2019 22:00
If a solution contains 3 moles/liter of sodium chloride (nacl, made of sodium ions and chloride ions), what is the osmolarity of this solution
Answers: 3

Chemistry, 22.06.2019 22:30
Which of the following molecules is polar? c3h7oh c2h5cooh
Answers: 1
You know the right answer?
Modeling Energy Changes Student Guide on Edge
Step 2: Write and Balance the Chemical Equation.
Questions

English, 04.01.2021 04:00




Mathematics, 04.01.2021 04:10








Geography, 04.01.2021 04:10

Mathematics, 04.01.2021 04:10

Mathematics, 04.01.2021 04:10


English, 04.01.2021 04:10

English, 04.01.2021 04:10

Mathematics, 04.01.2021 04:10

Advanced Placement (AP), 04.01.2021 04:10



