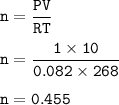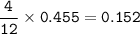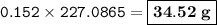Chemistry, 04.12.2020 14:00 littlemrslazy
Suppose that 10.0 L of Carbon Dioxide gas are produced by this reaction, 4C3H5N3O9 -> 12 CO2 + 10H2O + 6N2 +O2, at a temperature of -5 degrees C, and a pressure of exactly 1 atm. Calculate the mass of nitroglycerin that must have reacted in grams.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Y=‐1x + 7 if y has a value of ‐24 what is the value of x?
Answers: 1

Chemistry, 22.06.2019 00:30
Water (2510 g ) is heated until it just begins to boil. if the water absorbs 5.09×105 j of heat in the process, what was the initial temperature of the water?
Answers: 3

Chemistry, 22.06.2019 17:30
Oil rich countries in the middle east cover about 4% of earths total land area but prossess about 48% of the worlds known oil reserves what is the main reason for high concentration of reserves in this part of the world
Answers: 3

You know the right answer?
Suppose that 10.0 L of Carbon Dioxide gas are produced by this reaction, 4C3H5N3O9 -> 12 CO2 + 10...
Questions

English, 25.02.2021 19:00






Biology, 25.02.2021 19:00

Mathematics, 25.02.2021 19:00

Mathematics, 25.02.2021 19:00

Mathematics, 25.02.2021 19:00


Law, 25.02.2021 19:00

History, 25.02.2021 19:00

Mathematics, 25.02.2021 19:00

Mathematics, 25.02.2021 19:00

Mathematics, 25.02.2021 19:00

English, 25.02.2021 19:00