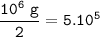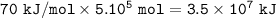Chemistry, 04.12.2020 14:00 ieyaalzhraa
In the reaction, 210 kJ of heat energy is used to form 3.0 moles of hydrogen. Calculate how much heat energy is needed to make 1000 kg of hydrogen.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:00
Which sentence about particles in matter is true? a. atoms are present in solids and liquids but not in gases. b. the particles of matter are in constant motion. c. the same kinds of atoms are found in different elements. d. when a solid changes to a liquid, the sizes of the particles change.
Answers: 1

Chemistry, 22.06.2019 17:30
What will most likely happen in the absence of a cell membrane? a) photosynthesis will not take place. b) the cell will not store food, water, nutrients, and waste. c) energy will not be released during cellular respiration. d) substances will pass in and out of the cell in an uncontrolled manner.
Answers: 1

Chemistry, 22.06.2019 18:00
Mercury turns to vapor at 629.88 k how much heat is lost 175 g of mercury vapor at 650 current condenses to a liquid at 297 ca mercury turns to weber at 629.88 kelvin how much he is lost 175 g of mercury vapor and 650 coming condensers to liquidate 297 kevin
Answers: 2

Chemistry, 23.06.2019 00:00
Exit what is the density of an object having a mass of 5.0 g and a volume of 45.0 cm3?
Answers: 1
You know the right answer?
In the reaction, 210 kJ of heat energy is used to form 3.0 moles of hydrogen.
Calculate how much he...
Questions


Health, 05.02.2021 22:30

Mathematics, 05.02.2021 22:30


Social Studies, 05.02.2021 22:30

Mathematics, 05.02.2021 22:30

Mathematics, 05.02.2021 22:30


Mathematics, 05.02.2021 22:30

Mathematics, 05.02.2021 22:30


Spanish, 05.02.2021 22:30


Mathematics, 05.02.2021 22:30

Mathematics, 05.02.2021 22:30


Mathematics, 05.02.2021 22:30



Mathematics, 05.02.2021 22:30