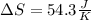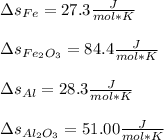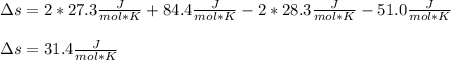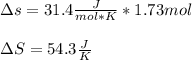Chemistry, 04.12.2020 16:30 jocelynmarquillo1
Fe2O3(s) 2Al(s)Al2O3(s) 2Fe(s) Using standard absolute entropies at 298K, calculate the entropy change for the system when 1.73 moles of Fe2O3(s) react at standard conditions.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:10
3.) for each of the following compounds, draw the major organic product of reaction with hcl or naoh and circle whether the starting materials and products will be more soluble in organic solvent or water benzoic acid + hcl: benzoic acid + naoh: oh benzoic acid water/organic water organic fluorenone hс: fluorenone + naoh: fluorenone water/organic water/organic веnzocaine + hci: benzocaine + n»oh: h2n benzocaine water/organic water organic o=
Answers: 3

Chemistry, 22.06.2019 13:00
Asubstance is a good conductor of electricity which of the following best explains a probable position of the substance in a periodic table
Answers: 3

Chemistry, 22.06.2019 14:00
How many absorptions would you expect to observe in the 13c nmr spectra of the following molecules? a) 3-chloropentane b) cis-4-methyl-2-pentene
Answers: 2

Chemistry, 22.06.2019 16:10
Predict the reactants of this chemical reaction. that is, fill in the left side of the chemical equation. be sure the equation you submit is balanced. (you can edit both sides of the equation to balance it, if you need to.) note: you are writing the molecular, and not the net ionic equation. > cacl2(aq) + h20(l)
Answers: 2
You know the right answer?
Fe2O3(s) 2Al(s)Al2O3(s) 2Fe(s) Using standard absolute entropies at 298K, calculate the entropy chan...
Questions



English, 16.09.2019 12:10


Chemistry, 16.09.2019 12:10





Mathematics, 16.09.2019 12:10

Business, 16.09.2019 12:10


Biology, 16.09.2019 12:10

Geography, 16.09.2019 12:10



English, 16.09.2019 12:10


Computers and Technology, 16.09.2019 12:10

History, 16.09.2019 12:10