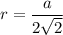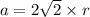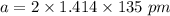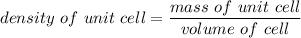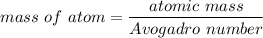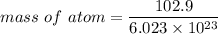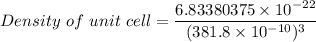Chemistry, 04.12.2020 17:00 SophomoreSareke
Rhodium crystallizes in a face-centered cubic unit cell. The radius of a rhodium atom is 135 pm. Determine the density of rhodium in g/cm3

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 11:00
The number to the right of an element's symbol (ex. c-12) identifies the of an isotope.
Answers: 1

Chemistry, 22.06.2019 12:30
The melting point of sulfur is 115 °c and its boiling point is 445 °c. what state would sulfur be in at 200 °c?
Answers: 1

Chemistry, 22.06.2019 16:00
About 3% of the water on earth is freshest. only about 40% of that freshwater is available for human use. why is so much freshwater unavailable for human use?
Answers: 2

Chemistry, 22.06.2019 18:30
Which of the following nuclei would be the least stable a 2 protons, 2 neutrons b 1 proton 1 neutron c 1 proton 3 neutrons d 1 proton 2 neutrons
Answers: 3
You know the right answer?
Rhodium crystallizes in a face-centered cubic unit cell. The radius of a rhodium atom is 135 pm. Det...
Questions



Computers and Technology, 28.09.2019 02:10

Mathematics, 28.09.2019 02:10





Mathematics, 28.09.2019 02:10




English, 28.09.2019 02:10

Mathematics, 28.09.2019 02:10



Mathematics, 28.09.2019 02:10