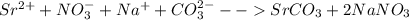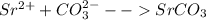Strontium compounds are often used in flares because their flame color is bright red. One industrial process to produce low-solubility strontiun) compounds (that are less affected by getting wet) involves the reaction of aqueous solutions of strontium nitrate and sodium carbonate. Write the balanced molecular equation, the total ionic equation and the net ionic equation for this reaction.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 11:00
The number to the right of an element's symbol (ex. c-12) identifies the of an isotope.
Answers: 1

Chemistry, 22.06.2019 21:00
Need what is special about water as a compound? how does water regulate climate? what drives water evaporation? why is the water vapor fresh water when it rises from the ocean? why might freshwater in the form of snow take longer to enter the water cycle again than liquid precipitation? what is an aquifer? what role do people play in the water cycle? plz just answer as many as you can ! thx if you !
Answers: 1

You know the right answer?
Strontium compounds are often used in flares because their flame color is bright red. One industrial...
Questions




Mathematics, 13.04.2021 01:30


French, 13.04.2021 01:30



Mathematics, 13.04.2021 01:30

Mathematics, 13.04.2021 01:30



Physics, 13.04.2021 01:30

Computers and Technology, 13.04.2021 01:30


Mathematics, 13.04.2021 01:30


Mathematics, 13.04.2021 01:30


Geography, 13.04.2021 01:30