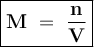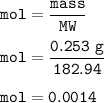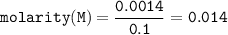Chemistry, 05.12.2020 03:10 Leggett8152
A chemist prepares a solution by adding 253 mg of Co(NO3)2 (MW = 182.94 g/mol ) to a volumetric flask, and then adding water until the total volume of the contents of the flask reaches the calibration line that indicates 100 mL . Determine the molarity of the prepared solution.

Answers: 2


Another question on Chemistry


Chemistry, 23.06.2019 03:00
Achemical equilibrium between gaseous reactants and products is shown. n2(g) + 3h2(g) ⇌ 2nh3(g) how will the reaction be affected if the pressure on the system is increased? it will shift toward the reactant side as there is lower pressure on the reactant side. it will shift toward the product side as there is higher pressure on the product side. it will shift toward the reactant side as there are a greater number of moles of gas on the reactant side. it will shift toward the product side as there are a fewer number of moles of gas on the product side.
Answers: 2

Chemistry, 23.06.2019 10:30
When a chemist collects hydrogen gas over water, she ends up with a mixture of hydrogen and water vapor in her collecting bottle. if the pressure in the collecting bottle is 97.1 kilopascals and the vapor pressure of the water is 3.2 kilopascals, what is the partial pressure of the hydrogen?
Answers: 1

You know the right answer?
A chemist prepares a solution by adding 253 mg of Co(NO3)2 (MW = 182.94 g/mol ) to a volumetric flas...
Questions

Mathematics, 23.03.2021 04:10

Mathematics, 23.03.2021 04:10


History, 23.03.2021 04:10


Social Studies, 23.03.2021 04:10


Mathematics, 23.03.2021 04:10


Mathematics, 23.03.2021 04:10


Chemistry, 23.03.2021 04:10





Mathematics, 23.03.2021 04:10



Mathematics, 23.03.2021 04:10