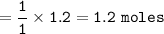If 1.2 moles of CuCl are mixed with
excess of H2S gas, how many moles of Cu2S would be made?...


Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
Un cierto gas tiene un volumen de 800ml a 80°c y 600ml a 80°c y 600mmhg de presión. ¿cual será el volumen del gas a condiciones normales? sí el gas es oxígeno, ¿cuál será su peso? y ¿cuántas moléculas están presentes en el sistema?
Answers: 2



Chemistry, 22.06.2019 09:40
Sulfur dioxide and oxygen react to form sulfur trioxide during one of the key steps in sulfuric acid synthesis. an industrial chemist studying this reaction fills a 25.0l tank with 4.5 mol of sulfur dioxide gas and 4.5 mol of oxygen gas at 30.°c. he then raises the temperature, and when the mixture has come to equilibrium measures the amount of sulfur trioxide gas to be 1.4 mol. calculate the concentration equilibrium constant for the reaction of sulfur dioxide and oxygen at the final temperature of the mixture. round your answer to 2 significant digits.
Answers: 3
You know the right answer?
Questions

Biology, 05.05.2020 04:45

Mathematics, 05.05.2020 04:45

Business, 05.05.2020 04:45



Mathematics, 05.05.2020 04:45

Engineering, 05.05.2020 04:45

Mathematics, 05.05.2020 04:45


Mathematics, 05.05.2020 04:45

Social Studies, 05.05.2020 04:45

Mathematics, 05.05.2020 04:45

Mathematics, 05.05.2020 04:45


Social Studies, 05.05.2020 04:45

History, 05.05.2020 04:45

Mathematics, 05.05.2020 04:45


Mathematics, 05.05.2020 04:45

Business, 05.05.2020 04:45