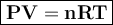Please help fast
A person breathes in 6.0 L of pure oxygen at 298 K and
1,000 kPa to fill the...

Please help fast
A person breathes in 6.0 L of pure oxygen at 298 K and
1,000 kPa to fill their lungs
How many moles of oxygen did they take in?
Use the ideal gas law: PV = nRT where R = 8.31 L – kPa /mol – K
A) 0.05 mole
B) 0.41 mole
C) 2.42 moles
D) 20.0 moles

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:30
Find the empirical formula of each of the following compounds. given mass or for each element in a sample of the compound 3,611 g ca; 6.389 g c1
Answers: 1

Chemistry, 22.06.2019 06:00
The tilt of the earth's axis of rotation is responsible for the a) ocean's tides. b) size of the moon. c) brightness of stars. d) earth’s seasons.
Answers: 1

Chemistry, 22.06.2019 06:30
The following reaction shows sodium carbonate reacting with calcium hydroxide. na2co3 + ca(oh)2 → naoh + caco3 how many grams of naoh are produced from 20.0 grams of na2co3? (molar mass of na = 22.989 g/mol, c = 12.01 g/mol, o = 15.999 g/mol, ca = 40.078 g/mol, h = 1.008 g/mol) 12.2 grams 15.1 grams 24.4 grams 30.2 grams
Answers: 2

You know the right answer?
Questions


English, 21.05.2020 05:08






Mathematics, 21.05.2020 05:08

Mathematics, 21.05.2020 05:08


History, 21.05.2020 05:08







Mathematics, 21.05.2020 05:08

English, 21.05.2020 05:08