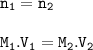Chemistry, 05.12.2020 04:10 huneymarie
A student has 0.75 L of a 5.0 M solution and dilutes it to make 3.5 liters. What’s the molarity of the diluted solution?
1.1 M
2.0 M
1.6 M
.93 M

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:30
The balanced chemical equation for this lab is: 3cucl2(aq) + 2al(s) 3cu(s) + 2alcl3(aq) if 10.5 g copper chloride react with 12.4 g aluminum, what is the limiting reactant?
Answers: 3

Chemistry, 21.06.2019 22:30
Using the periodic table, complete the table to describe each atom. type in your answers.a ? b? c? d? e? f?
Answers: 1

Chemistry, 22.06.2019 06:30
How many moles of carbon dioxide will form if 2.5 moles of c3h8 is burned
Answers: 1

Chemistry, 22.06.2019 08:00
Joe shines white light into a bowl half full of water at an angle of incident of 27.5°. calculate the angle of refraction in the water given the indices of refraction for air and water are 1.00 and 1.36, respectively.
Answers: 2
You know the right answer?
A student has 0.75 L of a 5.0 M solution and dilutes it to make 3.5 liters. What’s the molarity of t...
Questions

Mathematics, 17.11.2019 09:31

Computers and Technology, 17.11.2019 09:31

Mathematics, 17.11.2019 09:31


Mathematics, 17.11.2019 09:31

Health, 17.11.2019 09:31


Computers and Technology, 17.11.2019 09:31

Mathematics, 17.11.2019 09:31

Mathematics, 17.11.2019 09:31


Mathematics, 17.11.2019 09:31

Mathematics, 17.11.2019 09:31


English, 17.11.2019 09:31


Mathematics, 17.11.2019 09:31

Geography, 17.11.2019 09:31

Geography, 17.11.2019 09:31