
Chemistry, 05.12.2020 09:20 stephanieanaya7
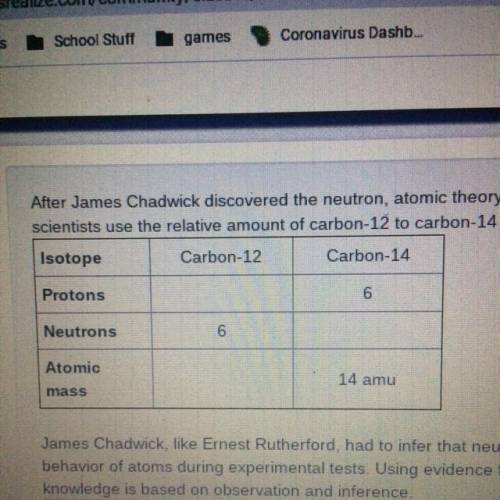
After James Chadwick discovered the neutron, atomic theory expanded to include isotopes. Isotopes have many practical uses. For example,
scientists use the relative amount of carbon-12 to carbon-14 in ancient bones to determine how old they are.
James Chadwick, like Emest Rutherford, had to infer that neutrons were present in atoms. He made inferences based on observations of the
behavior of atoms during experimental tests. Using evidence from Rutherford's gold foil experiment, defend the following claim: Scientific
knowledge is based on observation and inference

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 20:00
Listenbase your answer to the question on the information below.nuclear radiation is harmful to living cells, particularly to fast-growing cells, such as cancer cells and blood cells. an external beam of the radiation emitted from a radioisotope can be directed on a small area of a person to destroy cancer cells within the body.cobalt-60 is an artificially produced radioisotope that emits gamma rays and beta particles. one hospital keeps a 100.0-gram sample of cobalt-60 in an appropriate, secure storage container for future cancer treatment.which choice represents the correct product for the beta decay of the co-60? fe-60ni-60fe-61ni-61
Answers: 2

Chemistry, 22.06.2019 20:00
Glucose (c6h12o6) is an important biological molecule. (round the answer to nearest hundredth.) what is the percent by mass of carbon in glucose?
Answers: 2

Chemistry, 22.06.2019 21:00
Rays from the sun are not considered matter true or false
Answers: 2

Chemistry, 23.06.2019 03:00
Air pressure is measured in pascals. for a professional american football game, the ball should be inflated to about 90,000 pascals. scientists studied the effects of air temperature on the pressure inside american footballs by taking these steps: 1. prepare 100 footballs. 2. measure each football's air pressure. 3. divide footballs into 10 groups. 4. place the groups in different lockers cooled to different air temperatures. 5. after 12 hours, remove the footballs from lockers. 6. measure each football's pressure again. 7. compare the new pressures to the starting pressures. what two terms best describe the variable "air pressure inside the football" in this experiment? independent, qualitative independent, quantitative dependent, qualitative dependent, quantitative
Answers: 3
You know the right answer?
After James Chadwick discovered the neutron, atomic theory expanded to include isotopes. Isotopes ha...
Questions

Chemistry, 16.01.2020 01:31

English, 16.01.2020 01:31





Engineering, 16.01.2020 01:31








Social Studies, 16.01.2020 01:31


Health, 16.01.2020 01:31





