
Chemistry, 05.12.2020 17:20 ghari112345
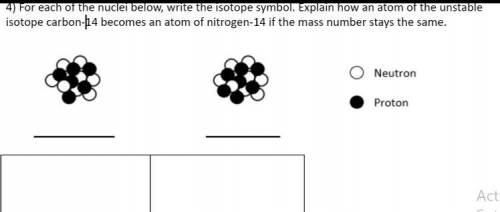
For each of the nuclei below write the isotope symbol. Explain how an atom of the unstable isotope carbon -14 becomes an atom of nitrogen 14 if the mass stays the same

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 12:00
An atom of which element reacts with an atom of hydrogen to form a bond with the greatest degree of polarity ?
Answers: 1

Chemistry, 22.06.2019 14:50
Which of the following is most likely true about water in chemical solutions?
Answers: 1

Chemistry, 22.06.2019 17:00
The arrangement of particles is most ordered in a sample of
Answers: 1

Chemistry, 23.06.2019 03:00
Abaker touches a pie right after taking it out of the oven. which statement best explains why the pie feels hot?
Answers: 1
You know the right answer?
For each of the nuclei below write the isotope symbol. Explain how an atom of the unstable isotope c...
Questions

Chemistry, 11.01.2021 19:10

History, 11.01.2021 19:10

Mathematics, 11.01.2021 19:10

History, 11.01.2021 19:10



Mathematics, 11.01.2021 19:10

Social Studies, 11.01.2021 19:10


Mathematics, 11.01.2021 19:10







Mathematics, 11.01.2021 19:10


Mathematics, 11.01.2021 19:10



