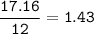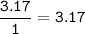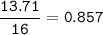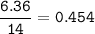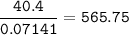Chemistry, 06.12.2020 05:00 timithythaxton
A 40.4 g sample of a protein contains 17.16 g of carbon, 3.17 g of hydrogen, 13.71 g of oxygen, and the rest being nitrogen. This 40.4-gram sample is known to be 0.07141 moles. Determine the molecular formula of this protein.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:30
An empty fuel tank can still contain and therefore can be even more dangerous than one full of liquid fuel.
Answers: 1

Chemistry, 22.06.2019 20:00
If one fission reaction of a uranium-235 atom produced two neutrons, how many neutrons would be released if the chain reaction occurred three more times?
Answers: 1

Chemistry, 22.06.2019 22:00
11) burning your hand when accidentally touching a hot plate is an example of which heat transfer? a. conduction b. convection c. radiation d. none of these
Answers: 2

Chemistry, 23.06.2019 01:30
Select the correct answer from each drop-down menu. to make a table of the elements, dmitri mendeleev sorted the elements according to their . he then split the list of elements into several columns so that elements beside each other had similar .
Answers: 2
You know the right answer?
A 40.4 g sample of a protein contains 17.16 g of carbon, 3.17 g of hydrogen, 13.71 g of oxygen, and...
Questions

Mathematics, 08.11.2019 06:31

Mathematics, 08.11.2019 06:31


Mathematics, 08.11.2019 06:31



Mathematics, 08.11.2019 06:31


Mathematics, 08.11.2019 06:31





Mathematics, 08.11.2019 06:31

Mathematics, 08.11.2019 06:31

Mathematics, 08.11.2019 06:31




Mathematics, 08.11.2019 06:31